Where is Hydrogen Energy Found?
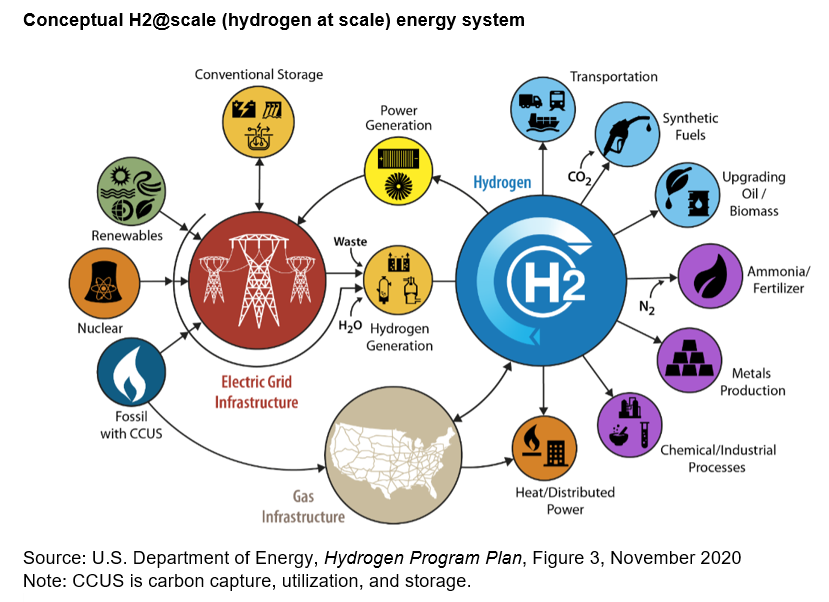
Where is Hydrogen Energy Found? https://h2-heat.eu/wp-content/uploads/2024/02/hydrogen_production_DOE.png 829 602 H2Heat Project https://h2-heat.eu/wp-content/uploads/2024/02/hydrogen_production_DOE.pngHydrogen energy is not found in a natural state like fossil fuels such as coal, oil, or natural gas. Instead, hydrogen is produced through various methods, and it can be derived from a variety of sources. Here’s a breakdown of where hydrogen energy comes from:
Production from Water (Electrolysis):
Hydrogen can be generated through a process known as electrolysis, which involves splitting water molecules (H2O) into hydrogen (H2) and oxygen (O2) using electricity. In this method, an electrical current is passed through water, causing it to undergo a chemical reaction that separates the hydrogen and oxygen atoms.
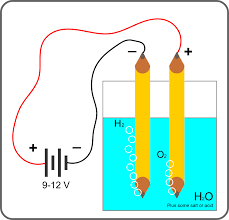
How it Works:
- Electrolysis Setup:
- A container of water is equipped with electrodes, typically made of materials like platinum or graphite, which serve as conductors for the electrical current.
- Application of Electricity:
- When electricity is applied to the electrodes, it energizes the water molecules, causing them to break apart into hydrogen and oxygen ions.
- Hydrogen and Oxygen Separation:
- The hydrogen ions (H+) are attracted to the negatively charged electrode (cathode), where they gain electrons to form hydrogen gas (H2).
- Simultaneously, the oxygen ions (O2-) are drawn to the positively charged electrode (anode), where they lose electrons to form oxygen gas (O2).
- Collection of Gases:
- The resulting hydrogen and oxygen gases are collected separately, typically in gas chambers, where they can be stored or utilized for various applications.
Environmental Considerations:
- Renewable Energy Source:
- To ensure environmental sustainability, electrolysis for hydrogen production often relies on electricity from renewable energy sources like solar, wind, or hydroelectric power. By using clean energy sources, the process minimizes greenhouse gas emissions and reduces environmental impact.
- Carbon Footprint Reduction:
- Electrolysis powered by renewable energy helps reduce carbon emissions associated with hydrogen production, making it an environmentally friendly alternative to conventional methods that rely on fossil fuels.
Advantages:
- Clean Energy Generation:
- Electrolysis offers a clean and sustainable method for producing hydrogen, as it does not produce any greenhouse gases or harmful pollutants during the process.
- Resource Availability:
- Water, the primary raw material for electrolysis, is abundant and readily available, making electrolysis a potentially scalable and cost-effective solution for hydrogen production.
- Versatility:
- Electrolysis can be conducted on various scales, ranging from small-scale applications for research or experimentation to large-scale industrial operations for commercial hydrogen production.
Challenges:
- Energy Efficiency:
- Electrolysis processes may require significant amounts of electricity, and improving energy efficiency remains a challenge to enhance the economic viability of large-scale electrolysis operations.
- Cost Considerations:
- The cost of electrolysis equipment and infrastructure can be relatively high, although ongoing advancements and increased adoption of the technology may lead to cost reductions over time.
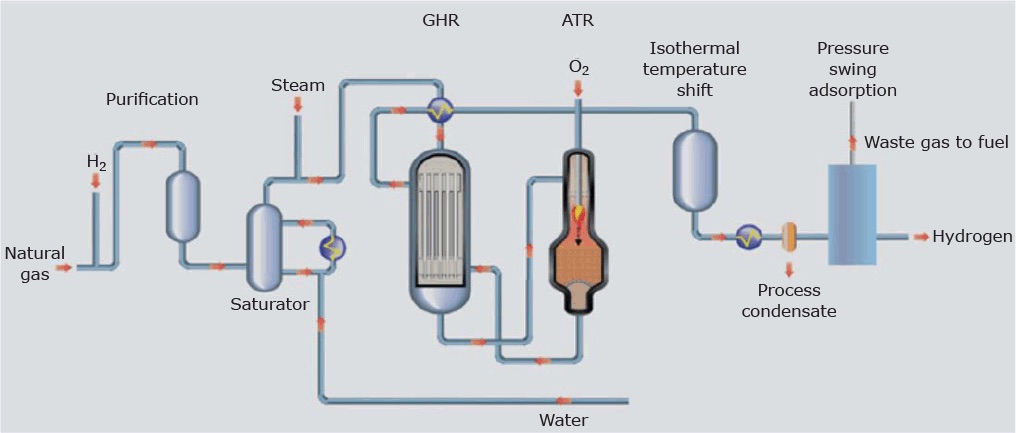
Production from Fossil Fuels (Steam Methane Reforming):
Steam methane reforming (SMR) is the predominant method of hydrogen production globally, particularly in industrial settings. In this process, natural gas (methane – CH4) undergoes a chemical reaction with steam at high temperatures to produce hydrogen (H2) and carbon dioxide (CO2).
How it Works:
- Chemical Reaction:
- Methane (CH4) from natural gas reacts with steam (H2O) in the presence of a catalyst, typically nickel, at temperatures ranging from 700 to 1,000 degrees Celsius.
- Hydrogen Production:
- The reaction between methane and steam produces hydrogen gas (H2) and carbon monoxide (CO), which then undergo a secondary reaction to form additional hydrogen and carbon dioxide.
- Carbon Dioxide Generation:
- While hydrogen is the desired product, the process also generates carbon dioxide (CO2) as a byproduct. This CO2 emissions are a significant environmental concern, contributing to climate change and global warming.
Environmental Considerations:
- Carbon Emissions:
- Steam methane reforming is associated with significant carbon emissions due to the release of CO2 during the production process. Without mitigation measures, these emissions contribute to climate change and environmental degradation.
- Carbon Capture and Storage (CCS):
- To mitigate the environmental impact of SMR, carbon capture and storage (CCS) technology can be employed to capture CO2 emissions and prevent them from entering the atmosphere. CCS involves capturing CO2 at the source, compressing it, and transporting it to underground storage sites, where it is permanently sequestered.
Advantages:
- Efficiency:
- SMR is a highly efficient method of hydrogen production, capable of yielding large quantities of hydrogen with relatively high purity.
- Established Infrastructure:
- Steam methane reforming benefits from well-established infrastructure and technologies, making it a cost-effective and reliable method of hydrogen production in industrial settings.
Challenges:
- Environmental Impact:
- The primary drawback of steam methane reforming is its significant carbon footprint due to CO2 emissions. Without effective mitigation measures, SMR contributes to greenhouse gas emissions and climate change.
- Dependence on Fossil Fuels:
- SMR relies on natural gas as a feedstock, perpetuating dependence on fossil fuels and hindering progress towards a sustainable energy future.
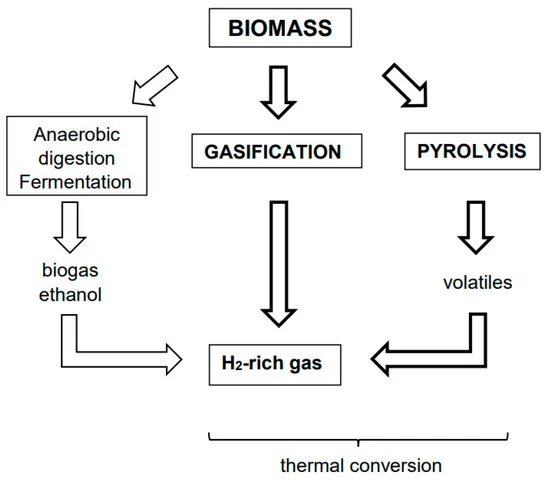
Biomass Conversion:
Hydrogen production from biomass offers a sustainable and renewable alternative to fossil fuel-based methods. Through processes such as gasification or pyrolysis, biomass materials such as organic waste, agricultural residues, or dedicated energy crops are transformed into hydrogen-rich gases through thermal or chemical reactions.
How it Works:
- Gasification:
- In gasification, biomass feedstocks are subjected to high temperatures and controlled amounts of oxygen, steam, or air in a gasifier. This thermochemical process breaks down the biomass into syngas, a mixture of hydrogen, carbon monoxide, methane, and other gases.
- Pyrolysis:
- Pyrolysis involves heating biomass in the absence of oxygen to produce a mixture of gases, liquids, and solids. The resulting pyrolysis gas, also known as bio-oil or synthesis gas, contains hydrogen along with other hydrocarbons and can be further processed to extract hydrogen.
Environmental Considerations:
- Renewable Feedstock:
- Biomass feedstocks are renewable resources derived from organic materials, offering a sustainable alternative to finite fossil fuel sources.
- Carbon Neutrality:
- Hydrogen production from biomass is considered carbon-neutral or even carbon-negative when sustainable feedstocks are utilized. This is because the carbon dioxide emitted during biomass combustion or gasification is offset by the carbon dioxide absorbed during biomass growth, resulting in no net increase in atmospheric CO2 levels.
Advantages:
- Resource Availability:
- Biomass resources are abundant and widely available, offering a readily accessible feedstock for hydrogen production.
- Waste Utilization:
- Biomass conversion processes can utilize organic waste streams, agricultural residues, and other biomass materials that would otherwise be discarded, providing a valuable means of waste management and resource recovery.
Challenges:
- Feedstock Logistics:
- The logistics of procuring and transporting biomass feedstocks can pose challenges, particularly in rural or remote areas where biomass resources may be limited or dispersed.
- Technology Development:
- Further research and development are needed to optimize biomass conversion technologies and improve efficiency, cost-effectiveness, and scalability.
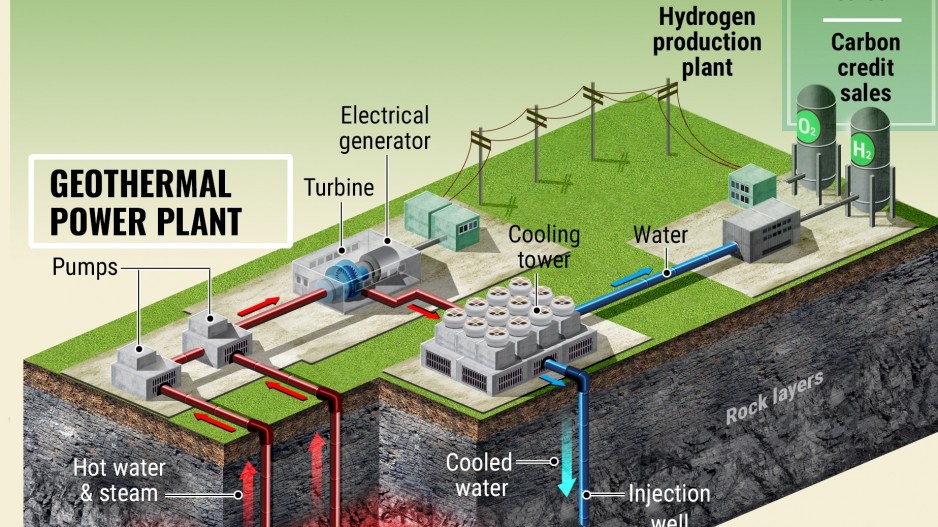
Other Renewable Sources:
In addition to water electrolysis, hydrogen can be produced from a variety of renewable sources, offering sustainable alternatives to fossil fuel-based methods. These renewable sources include biomass, geothermal energy, and excess electricity generated from renewable sources such as solar and wind.
Geothermal Energy:
- Hydrogen Extraction:
- Geothermal energy, derived from the Earth’s heat, can be used to extract hydrogen from water through geothermal electrolysis.
- By utilizing heat from geothermal reservoirs, this method offers a renewable and continuous source of energy for hydrogen production.
Environmental Considerations:
- Carbon Footprint Reduction:
- Utilizing renewable sources for hydrogen production helps reduce carbon emissions and environmental impact, contributing to efforts to mitigate climate change and transition to a low-carbon economy.
Advantages:
- Diversification of Energy Sources:
- By tapping into a diverse range of renewable sources, hydrogen production can become less reliant on fossil fuels, enhancing energy security and resilience.
- Sustainability:
- Renewable hydrogen production methods offer sustainable alternatives to finite fossil fuel resources, supporting the transition to a clean and renewable energy future.
Challenges:
- Technology Integration:
- Integration of renewable hydrogen production technologies into existing energy systems requires further research and development to optimize efficiency, scalability, and cost-effectiveness.
- Intermittency:
- Variability in renewable energy sources like solar and wind power can pose challenges for continuous hydrogen production, requiring innovative solutions for energy storage and grid integration.

Industrial Processes:
Hydrogen is a byproduct of various industrial processes, including petrochemical refining, ammonia production, and steel manufacturing. These industries produce hydrogen as a result of chemical reactions or as a byproduct of other processes. Capturing and utilizing this hydrogen can contribute to overall hydrogen supply, providing additional sources of this valuable energy carrier.
Petrochemical Refining:
- Hydrogen Generation:
- During petrochemical refining, hydrogen is often produced through processes such as catalytic reforming or hydrocracking. These processes generate hydrogen as a byproduct, which can be captured and utilized for various applications.
Ammonia Production:
- Byproduct Hydrogen:
- Ammonia production relies on the Haber-Bosch process, which involves reacting nitrogen and hydrogen to produce ammonia (NH3). Hydrogen is a key component of this process and is typically produced through steam methane reforming or electrolysis.
Steel Manufacturing:
- Hydrogen as Reducing Agent:
- In steel manufacturing, hydrogen is used as a reducing agent in processes such as direct reduction of iron ore. During these processes, hydrogen is consumed in chemical reactions, but it can also be captured and reused for other purposes.
Utilization:
- Feedstock:
- Captured hydrogen from industrial processes can be used as a feedstock for various applications, including ammonia production, methanol synthesis, and hydrocracking in refineries.
- Fuel Source:
- Hydrogen produced as a byproduct of industrial processes can also be used as a fuel source for power generation, heating, or transportation, providing an additional revenue stream and reducing emissions.
Biological Processes:
Certain microorganisms, such as algae or bacteria, have the ability to produce hydrogen through biological processes like photosynthesis or fermentation. While still in the research stage, biological hydrogen production holds potential for sustainable and environmentally friendly hydrogen production in the future.
Photosynthesis:
- Algae and Cyanobacteria:
- Some species of algae and cyanobacteria have the ability to produce hydrogen through photosynthesis, using sunlight as an energy source to split water molecules and release hydrogen gas as a byproduct.
Fermentation:
- Microbial Fermentation:
- Certain bacteria, such as Clostridium species, can produce hydrogen through fermentation of organic compounds. This process involves breaking down organic matter in the absence of oxygen, producing hydrogen and other byproducts.
Potential Benefits:
- Renewable and Sustainable:
- Biological hydrogen production offers a renewable and sustainable approach to hydrogen production, utilizing natural processes and resources.
- Environmental Benefits:
- Biological processes for hydrogen production have the potential to be environmentally friendly, generating minimal or no greenhouse gas emissions compared to traditional fossil fuel-based methods.
Challenges:
- Efficiency and Scalability:
- One of the key challenges in biological hydrogen production is improving efficiency and scalability to make the process economically viable on a large scale.
- Research and Development:
- Further research and development are needed to optimize biological hydrogen production methods, including strain engineering, cultivation techniques, and process optimization.